Modernas Vaccin / Ey7q 9qyyw Fim
Modernadid seek to create a booster shot for. Information about the Moderna COVID-19 vaccine including name manufacturer type of vaccine.
Since the COVID vaccines from Moderna Pfizer and Johnson Johnson are all quite new there arent years of data on them to prove how safe and effective they are over long.
Modernas vaccin. Modernas vaccine is administered as two 100-microgram doses given 28 days apart. However if it is not possible to follow the recommended interval you may schedule the second dose of the Moderna COVID-19 Vaccine for administration up to 6 weeks 42 days after the first dose. Information about the Moderna COVID-19 Vaccine.
In clinical trials approximately 15400 individuals 18 years of age and older have received at least 1 dose of the Moderna. Its advanced stage clinical trial started July 27 and it was the first government-funded. The Moderna shot is a messenger RNA mRNA vaccine that teaches our cells to make a piece of SARS-CoV-2 protein and mount an immune response against it.
You should administer the second dose as close as possible to the recommended interval of 28 days after dose 1. Similarly in Minnesota last month the authors found that the Moderna vaccine also known as mRNA-1273 was 76 effective at preventing infection but the Pfizer vaccine. General side effects.
Smiths Food and Drug has announced it will begin administering the Moderna COVID-19 vaccine for people 70 years old and older starting on Thursday February 11 2021 at. Find Educational Resources For You And Your Patients Today. The Pfizer-BioNTech and Moderna.
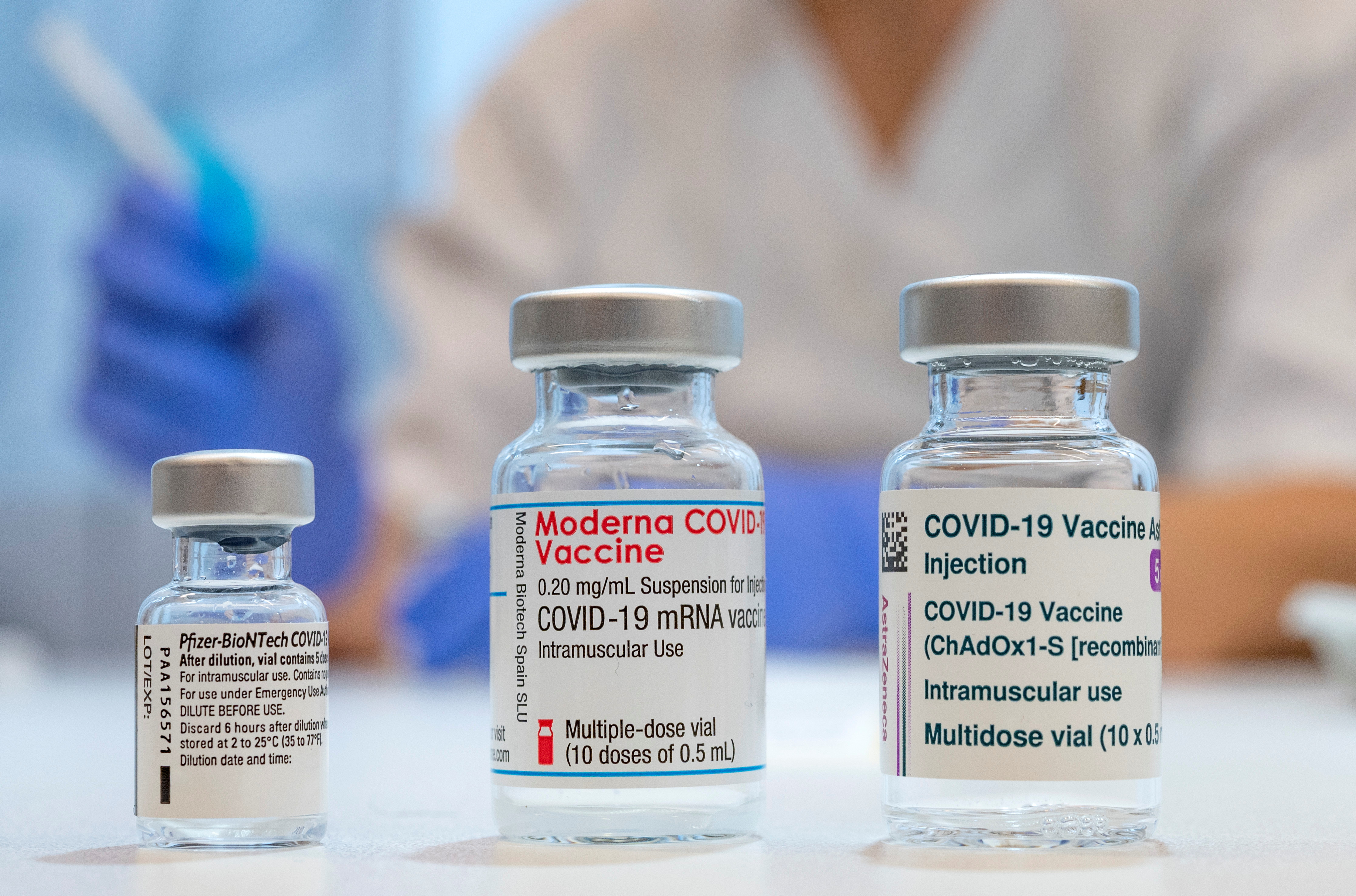
The Moderna COVID19 Vaccine has not been approved or licensed by the US Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency. FDA could authorize Moderna COVID booster vaccine at a half dose. The Moderna COVID-19 vaccine is an mRNA vaccine that requires 2 shots 28 days apart.
Ad Learn about Emergency Use Authorization EUA and why it was granted. The Moderna COVID19 Vaccine has not been approved or licensed by the US Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency. Modernas Covid-19 vaccine protects people for at least six months and likely longer -- even against new variants researchers reported Thursday.
Fatigue headache muscle pain joint pain chills nausea and vomiting and fever. Get the vaccine details. MRNA vaccines do not affect or interact with our DNA in any way.
Learn about safety data efficacy and clinical trial demographics. Ad Visit The Site And Get More Information To Help Answer Your Patients Questions. Moderna a Massachusetts-based vaccine developer partnered with the National Institutes of Health to develop and test a coronavirus vaccine.
Download the Moderna COVID-19 Vaccine fact sheet for more information. Ad Visit The Site And Get More Information To Help Answer Your Patients Questions. There are currently limited data on effectiveness of mRNA COVID-19 vaccines.
If necessary the interval between the doses. The Pfizer COVID-19 vaccine is significantly less effective against the delta variant than the Moderna vaccine. On December 18 2020 the US.
There is a remote chance that the Moderna COVID-19 Vaccine could cause. SAGE recommends the use of the Moderna mRNA-1273 vaccine at a schedule of two doses 100 µg 05 ml each 28 days apart. A head-to-head study of coronavirus vaccines in the United States finds the Moderna vaccine is slightly more effective than Pfizers in real-life use in keeping people out of.
The Moderna COVID-19 Vaccine is an unapproved vaccine. Ad Learn about Emergency Use Authorization EUA and why it was granted. Find Educational Resources For You And Your Patients Today.
How Modernas Vaccine Works. Download the Moderna COVID-19 Vaccine fact sheet for more information. The FDA green-lit clinical trials of Modernas vaccine on March 3 the first out of the gate.
The Moderna COVID-19 vaccine is a two-dose vaccine to prevent COVID-19. This Snapshot feature looks at the possible side effects and safety recommendations associated with this mRNA vaccine. Ad mRNA vaccines are a new type of vaccine to protect against infectious diseases.
Food and Drug Administration issued an emergency use authorization EUA for the second vaccine. If you got the Moderna vaccine against COVID-19 you could be in line for a booster shot.

Https Photos Lci Fr Images 613 344 Moderna Flacon Afp 874d30 0 1x Jpeg
Covid 19 Le Vaccin Moderna Approuve Par L Agence Europeenne Des Medicaments Et Bruxelles L Irlande Ferme Ses Ecoles

Risque Pour La Sante La Suede Suspend Le Vaccin Moderna Pour Les Moins De 30 Ans Tribune De Geneve
Https Static Latribune Fr 1436818 Coronavirus Resultats Prometteurs Pour Un Vaccin Experimental De Moderna Jpg

Https Images Laprovence Com Media Relaxnews 2021 05 2021 05 25 000 9aq2a9 D67de133438 W768 Jpg Twic V1 Focus 384x230 5 Cover 740x416

Https Static Lexpress Fr Medias 12278 W 2048 2ch 1146 2cc Crop 2cx 0 2cy 68 W 640 2ch 360 2cc Fill 2cg North V1618062918 Le Gouvernement Americain A D Ores Et Deja Achete 200 Millions De Doses Du Vaccin De Moderna 6286636 Jpg

Https Www Leparisien Fr Resizer Qosnlmyj7zfgwtj92mfwac3yqtq 932x582 Cloudfront Eu Central 1 Images Arcpublishing Com Leparisien Pl2ly3q3j5et3euiksoyskudje Jpg
Moderna Demande A Sante Canada D Autoriser Son Vaccin En Dose De Rappel Coronavirus Radio Canada Ca

Https S France24 Com Media Display Cc8c36b0 Ebbe 11eb B6a1 005056a97e36 000 9fq7pg Jpg

Https Www Capital Fr Imgre Fit Https 3a 2f 2fi 2epmdstatic 2enet 2fcap 2f2021 2f08 2f29 2fc2b9b4b7 D2ff 4411 9499 82ec71246f80 2ejpeg 768x432 Background Color Ffffff Focus Point 1159 2c612 Quality 70 Covid 19 La Finlande Suspend A Son Tour Le Vaccin Moderna 1416565 Jpg
Sweden To Give 12 15 Year Olds Pfizer Vaccine Rejects Moderna Reuters

Moderna Vers Un Vaccin Combine Covid 19 Grippe Le Point

Https Photos Lci Fr Images 613 344 Vaccin Moderna 696c22 0 1x Jpeg

Https Www Leparisien Fr Resizer 0 9rhc Ls Y5ii6qwipo335wbky 932x582 Cloudfront Eu Central 1 Images Arcpublishing Com Leparisien Vlmjsmfjkfdnllvglp5opoqby4 Jpg